FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology
The journal is the first and most reputable in Russia and EurAsEC (Eurasian Economic Community) countries peer-reviewed periodical that publishes materials on new medical technologies, economic optimization of drug therapy, quality-of-life and healthcare problems.
The journal was founded in 2008.
The impact factor of this journal, as shown in the Russian Science Citation Index (RSCI) is the highest among the periodicals in the areas of pharmacoeconomics, health technology assessment, and epidemiology. According to RSCI, the biennial impact factor (without self-citations) was 0.325 in 2013, 0.411 in 2014, and 0.722 in 2015.
The journal publishes various materials on pharmacoeconomics and pharmaco-epidemiology including the methodology, data analysis and results of studies on public health, medical technologies and economic aspects of drug therapies. The original articles and literature reviews cover Cost-of-Illness Analysis, Cost-Minimization Analysis, Cost-Effectiveness Analysis (CEA), Cost-Utility Analysis (CUA), Cost-Benefit Analysis (CBA), Quality of Life Assessment (QoL), Patients' Preferences & Patients’ Satisfaction indices and related topics.
Our aims and priorities focus on scientific and information support to the decision-makers and experts in public drug supply, health providers, research and education professionals, as well as pharmaceutic and insurance companies.
Languages: Russian, English
Periodicity: 4 issues per year (quarterly).
Copies of this journal are distributed under the Creative Commons Attribution 4.0 License: full-text materials are freely available to the public in an open access repository.
Distribution of the printed version: Russia, the EurAsian Economic Community countries (Belarus, Kazakhstan, Kyrgyzstan, Tajikistan, Uzbekistan, Armenia, Moldova)
The editorial board of “FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology” includes leading experts in pharmaco-economics, clinical pharmacology, medical technology assessment, epidemiology, and public health from Russia, USA and Spain.
The editorial board maintains the policy of full compliance with all principles of publishing ethics. Our ethical standards and codes conform to those of top international science publishers.
All submitted materials undergo a mandatory double-blind peer review.
Media Certificate of Registration: ПИ №ФС77-32713 of August 01, 2008.
ISSN 2070-4909 (Print)
ISSN 2070-4933 (Online)
By the decision of the Higher Attestation Commission (HAC) of Russia, “FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology” is included in the "List of top peer-reviewed scientific journals and publications" where scientists seeking academic degrees are required to publish their results.
The journal appears in the Russian Universal Scientific Electronic Library (RUNEB) elibrary.ru and is also present in the database of the Russian Science Citation Index (RSCI). Concise versions of major articles from this journal are published by the All-Russian Institute for Scientific and Technical Information (VINITI). The journal is also indexed by "Ulrich's periodicals Directory" – a global information system of periodicals and continued publications.
Current issue
ORIGINAL ARTICLES
What is already known about thе subject?
► When treating cutaneous melanoma (CM), ipilimumab + nivolumab combination is widely used, demonstrating good effectiveness. However, this approach is associated with high costs and unfavorable safety profile, which justifies the search for alternative solutions
► Previously, indirect comparisons of the efficacy and safety of nurulimab + prolgolimab combination against alternatives were conducted; however, the respective pharmacoeconomic studies data were lacking
What are the new findings?
► For the first time, a cost-effectiveness analysis for the use of nurulimab + prolgolimab combination was conducted across a cohort of patients with unresectable or metastatic CM
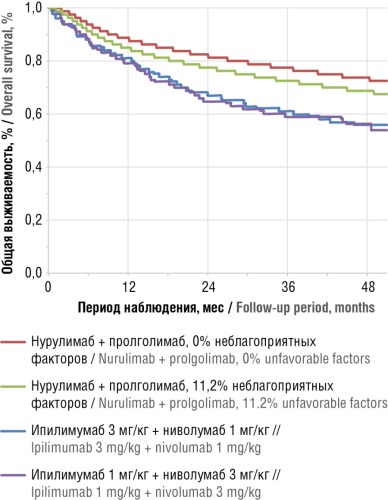
► In terms of pharmacoeconomic indicators, the nurulimab + prolgolimab combination has shown superior efficacy over the ipilimumab + nivolumab combination in various dosing regimens
How might it impact the clinical practice in the foreseeable future?
► The widespread introduction of nurulimab + prolgolimab combination will allow for a more rational use of healthcare resources due to comparable or lower costs while increasing the overall survival rates of CM patients and having a more favorable safety profile
Objective: To conduct a cost-effectiveness analysis of first-line treatment regimens based on nurulimab + prolgolimab combination in comparison with ipilimumab + nivolumab combination among adult patients in the Russian Federation with unresectable or metastatic cutaneous melanoma (CM).
Material and methods. An adjusted indirect comparison of the efficacy of nurulimab + prolgolimab combination and ipilimumab + nivolumab combination in both dose regimens was conducted according to data from BCD-217-2/OCTAVA and CheckMate 511 randomized controlled trials, respectively. Only direct medical costs for the first-line immunotherapy of unresectable or metastatic CM were taken into account, i.e. manufacture prices with 10% value-added tax and 5% discount rate. The cost-effectiveness analysis was based on estimation of cost per life years gained (LYG) and patient life preservation for a period from 1 to 5 years. Sensitivity analysis was used to assess the impact of price fluctuations on the study results.
Results. The total direct costs over 5-year horizon for the nurulimab + prolgolimab combination were 19% lower than that for the ipilimumab 3 mg/kg + nivolumab 1 mg/kg combination, and nurulimab + prolgolimab costs were comparable with the ipilimumab 1 mg/kg + nivolumab 3 mg/kg combination. At the same time, the cost per 1 LYG for the nurulimab + prolgolimab combination therapy turned out to be minimal, amounting to 3.07 million rubles versus 3.44–4.21 million rubles for ipilimumab + nivolumab combinations. In addition, cost per patient life preservation for the nurulimab + prolgolimab combination was lower than that for the ipilimumab 3 mg/kg + nivolumab 1 mg/kg therapy in the first year by 0.7 million rubles (10%), with this difference amounting to 9.1 million rubles (49%) by the fifth year. In comparison with the combination of nurulimab + prolgolimab, the costs for the ipilimumab 1 mg/kg + nivolumab 3 mg/kg combination required to reach patient life preservation in the first year was lower by 1.5 million rubles (20%), although increasing by 4.1 million rubles (23%) vs nurulimab + prolgolimab costs by the fifth year of therapy.
Conclusion. Widespread introduction of the nurulimab + prolgolimab combination for unresectable or metastatic CM into current clinical practice will increase patient survival and will contribute to achieving the target indicators of the Russian Ministry of Health in terms of reducing one-year mortality and increasing the proportion of patients with malignant neoplasms registered for 5 or more years. This effect can be achieved in the setting of lower or comparable costs of treatment, which allows improved cost-effectiveness to be achieved when applying the prolgolimab + nurulimab combination.
What is already known about thе subject?
► Experimental data support the hypothesis that gluten, as an exogenous antigen, induces the formation and subsequent mesangial deposition of IgA-containing immune complexes, thus validating the concept of an enterorenal axis in IgA nephropathy (IgAN) pathogenesis
► Seropositivity for IgA antibodies to deamidated gliadin peptides (anti-DGP IgA) is associated with a more severe course of IgAN, characterized by significant proteinuria, arterial hypertension, and a high risk of progression
What are the new findings?
► The findings demonstrate a correlation between seropositivity for anti-DGP IgA and predominant fibrotic-sclerotic changes in kidney biopsies of patients. These patients thus have a morphological variant of IgAN, which is associated with an unfavorable prognosis for the disease
How might it impact the clinical practice in the foreseeable future?
► Targeted screening using a highly specific test for anti-DGP IgA is cost-effective, as it prevents unnecessary expenditure on comprehensive immunological workups for all IgAN patients
► The determination of anti-DGP IgA in IgAN patients holds significant prognostic value by enabling early detection of progression, risk stratification for adverse outcomes, and guidance for optimal treatment
Backgrоund. Immunoglobulin A nephropathy (IgA-N) is a common form of chronic glomerulonephritis. Its basis is the accumulation of IgA immune complexes in the glomeruli, associated with impaired glycosylation of the IgA molecule. Exogenous antigens, such as gluten, play an important role in the development of the disease. It has been experimentally confirmed that oral immunization with gluten induces mesangial IgA deposits, and a gluten-free diet can improve the course of nephropathy, indicating the significance of the enterorenal axis in the pathogenesis of IgA-N.
Objective: To study structural changes in renal tissue in IgAN patients and to evaluate the clinical and diagnostic significance of IgA antibodies to deamidated gliadin peptides (anti-DGP IgA) in the blood serum.
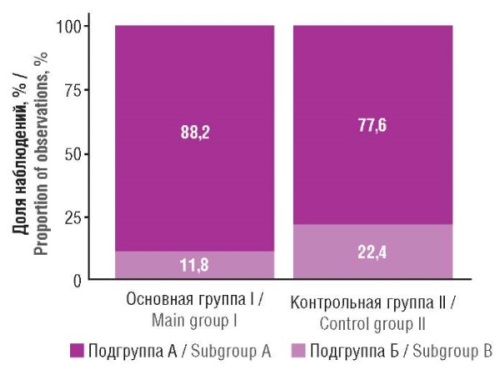
Material and methods. The study involved 105 patients aged 18 to 64 years diagnosed with IgAN based on a lifetime nephrobiopsy with morphological examination according to the Oxford classification. Patients were divided into two groups: the main group (n=20) included IgAN patients with detected anti-DGP IgA, and the control group (n=85) included IgAN patients seronegative for anti-DGP IgA, IgA antibodies to tissue transglutaminase, and to endomysium.
Results. When comparing fibrous-sclerotic changes, no statistically significant intergroup differences were obtained. However, a clear trend of predominance of irreversible light-optical changes within the area of the nephrobiopsy specimen was noted in patients of the main group compared to the control group: 82.4% and 56.9%, respectively (p=0.059).
Conclusion. The obtained results reflect a high frequency of irreversible fibrous-sclerotic changes in the nephrobiopsy in patients with IgAN and anti-DGP IgA in the blood serum. Timely detection of anti-DGP IgA can improve the ability to predict the outcome of the disease and optimize therapeutic strategies.
What is already known about thе subject?
► New technologies used in healthcare are represented by various software products (including systems for supporting medical decision-making), digital services, medical devices based on artificial intelligence, and other solutions, all grouped under the general term of digital health products and services (DHPS)
► The development and implementation of DHPS is a costly process; however, their benefits often remain underassessed from a clinical and economic point of view. This imposes limitations on the widespread adoption of DHPS
► The tools of health technology assessment (HTA) can help accelerate implementing DHPS. However, existing HTA methods were primarily developed for pharmaceuticals, with the experience of their application for DHPS remaining limited
What are the new findings?
► The fundamental applicability of methods used for assessing pharmaceuticals to DHPS was demonstrated
► Specific features of DHPS that should be considered in assessment were identified
► A draft dossier for DHPS was proposed. It contains the list of required information about the product in question and structures the data for subsequent assessment and decision-making
How might it impact the clinical practice in the foreseeable future?
► The results obtained can serve as the basis for further development and practical application of drug assessment methods to DHPS
Objective: To analyze the applicability of approaches conventionally used to assess the clinical and economic effectiveness of pharmaceuticals to that of digital health products and services (DHPS).
Material and methods. In this study, DHPS was considered to be an umbrella concept that encompasses various health technologies such as mobile health apps and services, including those based on artificial intelligence. Approaches to drug assessment were reviewed based on regulatory documents, methodological guidelines, and monographs on drugs assessment.
Results. The examined approaches to assessing the effectiveness and safety of pharmaceuticals can be applied to DHPS, having no fundamental limitations. Specific characteristics of DHPS that influence the choice of their assessment approaches were identified and described. A draft dossier for DHPS was proposed, with its composition aligned with the goals and objectives of the assessment task.
Conclusion. Future research should address the development of methodological guidelines for assessing DHPS based on the approaches used for drug assessment.
What is already known about thе subject?
► Treatment of neuroendocrine tumors (NETs) includes a wide range of approaches, such as surgery, targeted therapy, chemotherapy, radionuclide therapy, and somatostatin analog therapy
► Somatostatin analogs, particularly octreotide and lanreotide, are the cornerstone of therapy for patients with gastrointestinal NETs. Both drugs have proven to be effective in controlling hormone-dependent symptoms and inhibiting tumor growth
► The choice of an optimal strategy for NETs remains a subject of debate due to the heterogeneity of clinical manifestations, the degree of tumor differentiation, and the variability of treatment response. Pharmacoeconomic assessment of extended release formulations of somatostatin analogs remains relevant
What are the new findings?
► The average 10-year cost of lanreotide therapy per patient is 7,213,219.59 rubles, compared to 4,279,437.69 rubles for octreotide (a difference of 40.67%). The cost of treating adverse events is lower for lanreotide compared to octreotide, being 24,604.01 and 59,203.81 rubles (a difference of 58.4%)
► When calculating cost-effectiveness ratio, the cost per month of life extended with lanreotide therapy was 63,825.60 rubles, compared to 48,046.97 rubles with octreotide (a difference of 32,83%)
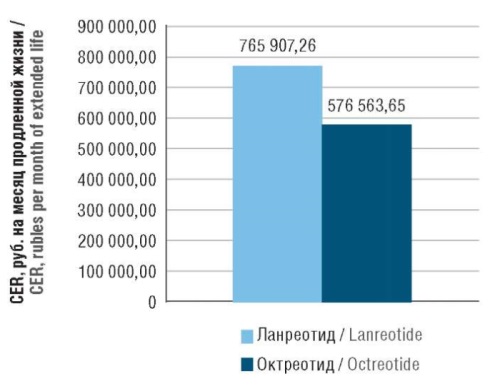
► When converted to annual costs, the cost per year of life extended with lanreotide therapy was 765,907.26 rubles, compared to 576,563.65 rubles using octreotide (a difference of 24.73%)
How might it impact the clinical practice in the foreseeable future?
► Although lanreotide is a more expensive medication than octreotide, its use may be justified by its greater therapeutic efficacy and convenient administration regimen, which contributes to increased treatment adherence
► The incremental cost-effectiveness ratio for lanreotide ranges within justifiable economic costs, being significantly below the willingness-to-pay threshold for the Russian Federation
► The results of the conducted pharmacoeconomic study have confirmed that the use of lanreotide in adult patients with gastrointestinal NETs is both a clinically justified and economically feasible choice in the Russian healthcare system
Objective: To conduct a comparative pharmacoeconomic assessment of the use of extended release formulations of lanreotide and octreotide in adult patients (over 18 years of age) with gastrointestinal neuroendocrine tumors (GI NETs) in the Russian healthcare system in 2025.
Material and methods. A comparative analysis of clinical trial data, including randomized controlled trials and retrospective cohort studies, was conducted on the efficacy and safety profiles of lanreotide and octreotide. The focus was on overall survival, progressionfree survival, and the incidence of adverse events. Next the study employed the methods involving the calculation of the costeffectiveness ratio (CER) and the incremental cost-effectiveness ratio (ICER), as well as sensitivity analysis. Economic parameters (costs, CER, ICER) are presented both in absolute values (rubles) and as relative measures (%).
Results. The average costs of drug therapy with lanreotide for 10 years per patient comprised 7,213,219.59 rubles, compared to 4,279,437.69 rubles for a course of extended release octreotide (a difference of 40.67%). At the same time, the costs of treating adverse events were lower for lanreotide compared to octreotide, equaling 24,604.01 and 59,203.81 rubles, respectively (a difference of 58.4%). Direct medical costs for a 10-year course of lanreotide therapy in one patient with GI NETs were 7,237,823.60 rubles, compared to 4,338,641.50 rubles for a course of octreotide, with the difference comprising 2,899,182.10 rubles (40.06%). When calculating CER, the cost of 1 month of extended life with lanreotide therapy was 63,825.60 rubles, compared to 48,046.97 rubles using octreotide (a difference of 32,83%). When recalculated to annual costs, the cost of 1 year of extended life with lanreotide therapy was 765,907.26 rubles, compared to 576,563.65 rubles using octreotide (a difference of 24.73%). ICER for lanreotide was 1,506,068.62 rubles per year, which is within the limits of justified economic costs, being significantly lower than the willingness to pay for the Russian Federation (about 3,350,000 rubles per year). Results of a one-way sensitivity analysis showed that the benefit of lanreotide is achieved with a 25% increase in the price of octreotide or a 33% increase in overall survival with lanreotide.
Conclusion. The use of lanreotide in adult patients with GI NETs is both a clinically justified and economically feasible choice in the context of Russian healthcare.
What is already known about thе subject?
► Nootropic drugs are used to improve cognitive functions, memory, and attention
► The demand for nootropics is growing due to intensified stress and increased brain disease incidence
► The nootropic drug market in Uzbekistan is actively developing, requiring detailed analysis
What are the new findings?
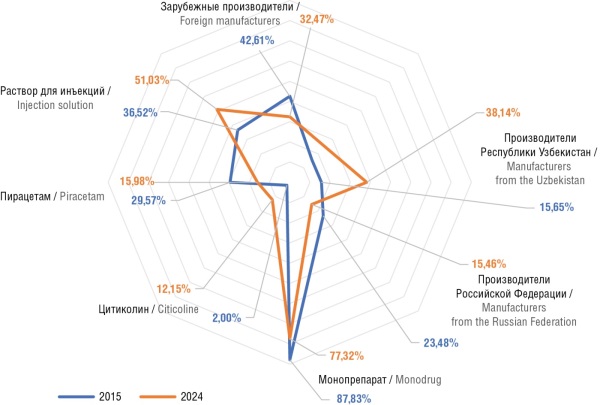
► The number of registered nootropic drugs in Uzbekistan increased from 112 in 2015 to 199 in 2024
► The share of domestic manufacturers has significantly increased, introducing new drug forms
► Citicoline, which has shown a notable increase in the number of registrations, is attracting attention
How might it impact the clinical practice in the foreseeable future?
► The data obtained indicate an increased accessibility of nootropic drugs for patients
► Wider use of citicoline and combination drugs improve the treatment effectiveness of cerebrovascular diseases
► Extended drug assortment of nootropics offers a wider choice of effective and affordable treatment options
Background. Nootropics are pharmacological agents that enhance such cognitive functions as memory and attention, as well as the overall mental performance. In recent years, global demand for these pharmaceuticals has significantly increased due to intensified psychological stress, a growing incidence of neurological disorders, and a growing public focus on mental health. In Uzbekistan, the nootropic drug market is experiencing active growth, underscoring the need for a comprehensive analysis of its structure and key trends.
Objective: To analyze trends in the nootropic drug market in Uzbekistan from 2015 to 2024, to identify key trends and factors influencing its development, and to assess the dynamics of supply and demand.
Material and methods. The available data on registered nootropic drugs in Uzbekistan, pharmaceutical company reports, sales statistics, and information on government policy and support measures were used. The dynamics of the number of registered trade names and their distribution by manufacturing country were studied. Leading companies playing a key role in the market were identified. The distribution of nootropics by composition (monodrugs and combination forms) and dosage forms (oral, injectable, etc.) was also examined.
Results. The analysis revealed a steady growth in the nootropic drug market in Uzbekistan throughout the study period. This growth is primarily driven by the increasing demand for cognitive enhancers across various age groups. One evident trend consists in the expanding presence of domestic manufacturers, who are introducing novel drug forms, including combination and injectable formulations. Additionally, innovative compounds, such as citicoline that has shown considerable therapeutic potential across various neurological conditions, are increasingly attracting attention.
Conclusion. The nootropic drug market in Uzbekistan is demonstrating positive growth dynamics, driven by rising consumer demand and reinforced by supportive government initiatives. The market is expected to continue to grow, with improvements in drug variety and accessibility. Both local and international pharmaceutical companies play a pivotal role in enhancing product quality and ensuring broad patient access.
What is already known about thе subject?
► Lymphoproliferative disorders are a predominant category of cancers related to human immunodeficiency virus (HIV)
► Comparative analysis reveals equivalent therapeutic efficacy for Hodgkin lymphoma between HIV-positive patients and immunocompetent subjects
► Treatment costs for HIV-related lymphomas remain a critical concern for healthcare systems, as comorbid opportunistic infections substantially increase the financial burden
What are the new findings?
► The first Russian analysis of the scale and cost structure for treating HIV-associated Hodgkin lymphoma was carried out
► The data facilitate comprehensive analysis of fundamental cost-affecting parameters, their variation tendencies under different scenarios, and associated probabilities
How might it impact the clinical practice in the foreseeable future?
► The presented data allow for broader implementation of pharmacoeconomic instruments to evaluate complex multi-component technologies in high-tech medicine
► The study results will help to optimize the set of pharmacoeconomic assessment methods for oncology-related health technologies
► Approaches to reduce treatment costs for hematologic malignancies were shown, particularly in immunocompromised patients, by implementing clinically and economically efficient technologies
Objective: To evaluate, from a pharmacoeconomic perspective, treatment strategies for adult patients with Hodgkin lymphoma (HL) complicated by human immunodeficiency virus (HIV) infection and HL patients without HIV infection.
Material and methods. The study sampled 67 adult HL patients including 34 HIV-positive (HIV-HL group) and 33 HIV-negative (nonHIV-HL group) subjects. All patients were treated at the Loginov Moscow Clinical Scientific Center between June 2015 and December 2023. The median surface area and body weight of patients were 1.85 m2 and 70.9 kg, respectively. Cost estimation was performed using decision-tree pharmacoeconomic modeling, medical institution price lists (excluding marginal markup). Government-regulated drug prices (for essential medicines), and 2024 public procurement prices (for other drugs, including targeted therapies and autologous stem cell transplantations) were considered. HIV treatment costs were excluded. Clinical endpoints included remission rates, 5-year overall survival, 5-year event-free survival, and 5-year relapse-free survival. Pharmacoeconomic analyses comprised cost-minimization, cost-effectiveness, and incremental cost-effectiveness analyses. Results were validated via probabilistic sensitivity analysis using Monte Carlo simulation (10,000-patient extrapolation).
Results. Treatment in the HIV-HL group was found to be more cost-effective both with the development of complications (1,020,332 vs. 3,935,166.50 rubles) and without complications (440,616 vs. 584,641 rubles), provided that the median cost of treatment is calculated. Weighted average costs favored HIV-HL treatment only with complications (2,416,961.67 vs. 3,578,882.08 rubles); without complications, non-HIV-HL treatment was more cost-effective (575,499.19 vs. 852,010.48 rubles). Monte Carlo simulations revealed model instability, though two of four scenarios (remission in HIV-HL with complications, using median and weighted costs) demonstrated robust outcomes.
Conclusion. The decision-tree model effectively evaluated median and weighted treatment costs for HL by HIV status. A combined pricing approach identified key cost drivers, including median-mean disparities, while cost-effectiveness and Monte Carlo analyses confirmed result stability. Both methods highlighted limitations in extrapolating small-cohort data to broader populations.
What is already known about thе subject?
► The tariff agreement (TA) serves as the main regulatory document governing the order of funding of medical services in a specific constituent entity of the Russian Federation. It establishes mechanisms for medical care coverage, tariffs, measures of financial responsibility for failure to fulfill obligations to provide such care within the territorial compulsory health insurance (CHI) program, as well as the scope of services and levels of their financial support
► Despite the requirements established by the Russian Ministry of Healthcare for the structure and content of TA, their form, presentation format, and level of detail are not regulated
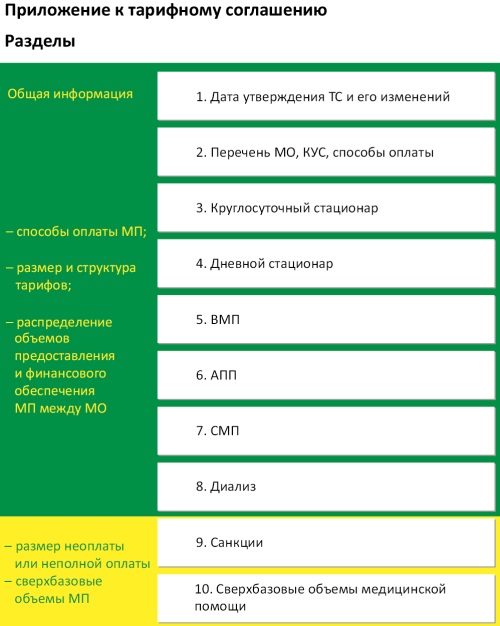
► A comparative analysis of TAs in 2022 revealed significant variations in their structure and level of detail, which complicates the analysis of information, reduces the transparency of regional tariff policy in the CHI system, and makes it difficult to assess the effectiveness of budget spending in healthcare
What are the new findings?
► A unified table format was created for regional TA, which will enable standardization of documentation, reduce the number of errors when filling out and checking forms, eliminate ambiguity in the interpretation of provisions, and significantly facilitate automated data processing
How might it impact the clinical practice in the foreseeable future?
► The use of standardized table formats plays a key role in ensuring data comparability between regions, implementing digital transformation, and organizing operational analytical monitoring. This approach is consistent with the current trends in healthcare system modernization and requirements for improving industry management efficiency
► The introduction of unified digital formats for TA creates a fundamentally new methodological basis for managing the Russian healthcare system, enabling an integrated effect. This includes complete transparency in pricing policy, operational monitoring of TA implementation, and automated control of the volume and quality of medical care in accordance with established standards
Background. At present, the compulsory health insurance (CHI) system in the Russian Federation is lacking a standardized format for tariff agreements. This leads to significant variability in the structure of documents and, consequently, to errors, low transparency of tariff policy, and financial control difficulties.
Objective: To develop a unified form of tariff agreement in the Russian CHI system.
Material and methods. The research methodology was based on an integrated analysis of the regulatory framework and regional tariff agreements, followed by testing of the developed form in pilot Russian regions.
Results. A unified form of tariff agreement has been developed. This standardized form minimizes the administrative burden on participants in the CHI system, ensures transparency in tariff formation, enables comparative analysis of regional indicators, and optimizes the process of document approval by the Federal CHI Fund.
Conclusion. The introduction of the standardized form for tariff agreements and the digitization of their processing will significantly improve the efficiency of tariff policy management in the CHI system of Russia.
What is already known about thе subject?
► Nonsteroidal anti-inflammatory drugs (NSAIDs), used as analgesics and anti-inflammatory agents, exert their primary effects by dramatically reducing the activity of the COX-2 and COX-1 cyclooxygenase enzymes
► Zinc, an important microelement, exhibits anti-inflammatory properties due to interactions with thousands of proteins in the human proteome
► Zinc-diclofenac and zinc-indomethacin complexes, as well as mixtures with organic zinc salts are characterized by a reduced incidence of gastric ulceration
What are the new findings?
► The chemoreactomic evaluation of the effect of zinc-imidazole complexes on prostaglandin metabolism showed them to be capable of inhibiting prostaglandin D2 binding to prostaglandin D2 receptor on platelets to a greater extent than zinc-NSAIDs
► The chemoreactomic analysis showed comparable effects of zinc-imidazole complexes and zinc-NSAIDs on COX-2 inhibition in cells and in whole blood
► The analysis of the effects of molecules on inflammation through cytokine mechanisms showed comparable effects of zinc-imidazole complexes and zinc-NSAIDs on the inhibition of bradykinin B1 and neurokinin-1 receptors
How might it impact the clinical practice in the foreseeable future?
► Zinc-imidazole complexes can inhibit COX-1 to a greater extent, which is important for reducing the side effects of NSAIDs, as well as having a more effective analgesic effect by displacing nociceptin from opiate-like receptors 1
► Zinc-containing compounds can serve as а basis for the development of new NSAIDs to improve the effectiveness and safety of pharmacotherapy for inflammation and pain
Background. Zinc-containing compounds are a promising basis for the development of new non-steroidal anti-inflammatory drugs (NSAIDs), which can raise the effectiveness and safety of pharmaceutical management of inflammation and pain.
Objective: To study the anti-inflammatory, ulcerogenic, etc., effects of zinc-imidazole complexes, such as allyl-2 (bis (N-allyl-2-methylimidazole) zinc diacetate), allim-2 (bis (N-allenyl-2-methylimidazole) zinc diacetate), and propargyl-2 (bis (N-propargyl-2-methylimidazole) zinc diacetate) in comparison with zinc complexes with known NSAIDs, such as diclofenac, nimesulide, and ketorolac.
Material and methods. In silico modeling of candidate molecules allyl-2, allim-2, propargyl-2, zinc-diclofenac, zinc-nimesulide, and zinc-ketorolac was performed using a toolkit of chemoinformatic analysis methods developed by scientific school of Yu.I. Zhuravlev and K.V. Rudakov through topological analysis of chemographs and numerical forecasting of distinguishing features of complex systems. These methods include the theory of chemograph analysis, methods for predicting numerical target variables, the combinatorial theory of solvability/regularity, and topological methods for data analysis. The pharmacological capabilities of molecules within the framework of chemoreactome methodology were evaluated by comparing the chemical structure of the query molecule with the structures of molecules whose molecular-pharmacological properties have been established and available in the PubChem, HMDB, STRING, and PharmGKB databases.
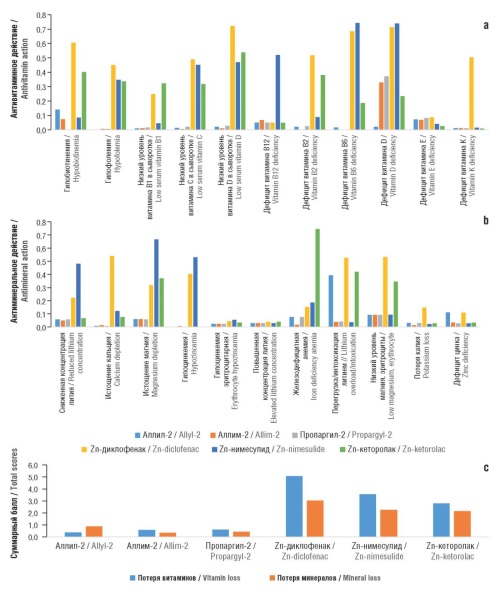
Results. The obtained chemoreactome evaluations revealed the capacity of zinc-imidazole complexes to inhibit of prostaglandin D2 binding to the prostaglandin D2 receptor on platelets (IC50 448–627 nM; zinc-NSAID: 588–997 nM) with comparable effects of zinc-imidazole complexes and zinc-NSAID on cyclooxygenase-2 (COX-2) inhibition in whole blood (IC50 295–428 nM). Zinc-imidazole complexes were characterized by a more pronounced inhibition of the proinflammatory signaling cascade of the NF-κB transcription factor (IC50 173–419 nM; zinc-NSAID 498–508 nM), alpha-1 adrenergic receptor (28 nM; zinc-NSAID: 235–411 nM), and angiotensin receptor-1 (IC50 16–22 nM; zinc-NSAID: 20–74 nM), indicating an antihypertensive effect. The antinociceptive activity of zinc-imidazole complexes (IC50 0.16 mg/kg) upon subcutaneous administration to mice in acetic acid-induced writhing was more pronounced than that of zinc-NSAIDs (0.9–1.0 mg/kg) with the exception of zinc-ketorolac (0.16 mg/kg). Compared to the zinc-NSAIDs, all zinc-imidazole complexes under study were characterized by similar and extremely low values of antimicronutrient action scores (antivitamin score 0.38–0.61, antimicroelement score 0.37–0.88; compared to two- or three-fold higher scores for zinc-NSAIDs), which indicates the absence of adverse effects of zinc-imidazole complexes on micronutrient metabolism.
Conclusion. The studied candidate molecules (zinc-imidazole complexes), in addition to COX inhibition, may exhibit additional pharmacological properties to a greater extent than the studied zinc complexes with known NSAIDs.
What is already known about thе subject?
► Most countries (European Union, Brazil, Japan, etc.) apply a well-established legal norm, which exclude from patent monopoly the individual compounding of medicinal products in pharmacies pursuant to a physician’s prescription
► The terms “medicinal product,” and “pharmaceutical substance” are interpreted differently in Russian law compared to international regulatory frameworks, creating terminological ambiguity when borrowing foreign legal norms
► Clause 5 of Article 1359 of the Civil Code of the Russian Federation (CC RF), introduced in 2006, was borrowed from European regulatory models but has not been adapted to Russia’s current pharmaceutical legislation
What are the new findings?
► Key legal inconsistencies between Clause 5 of Article 1359 of CC RF and the Federal Law “On medicinal products” were identified
► For the first time in Russian academic literature, a comparative legal
analysis of similar patent exceptions in 14 jurisdictions was conducted
► The need for legislative clarification of Clause 5 of Article 1359 of CC RF was substantiated to ensure legal certainty and compliance with public health objectives
How might it impact the clinical practice in the foreseeable future?
► Clarifying the norm will eliminate terminological ambiguity and reduce the risk of divergent interpretations of Clause 5 of Article 1359 of CC RF
► The provision of Clause 5 of Article 1359 of CC RF may serve as a negotiating tool for the state to ensure access to high-cost medicines during shortages or anti-competitive patent practices
Background. Identification of terminological and practical inconsistencies in the field of patent law in regulatory legal acts, including the Civil Code of the Russian Federation (CC RF) is important for lawmakers, regulators, stakeholders in the pharmaceutical market, and patent holders.
Objective: To conduct a comprehensive analysis of Clause 5 of Article 1359 of CC RF, which provides an exception to patent holders’ exclusive rights regarding the compounding of drugs in pharmacies pursuant to physicians’ prescriptions, in terms of its consistency with current pharmaceutical legislation and international legal standards.
Material and methods. The methods of historical-legal, semantic-linguistic, and comparative-legal analysis were applied. The Russian regulation was compared with that in the European Union (EU), the United States of America, Japan, Brazil, etc. Additionally, provisions of international agreements, including the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) and the EU Agreement on a Unified Patent Court, were analyzed.
Results. The current wording of Clause 5 of Article 1359 of CC RF was found to suffer from insufficient terminological clarity regarding the concepts of “individual compounding” and “medicinal product”, thus leading to legal uncertainty and potential conflicts with existing pharmaceutical legislation. The analysis of international practices revealed common regulatory approaches to this exception: the individual (personalized) nature of compounding (for a specific patient), mandatory medical prescription, exclusion of industrial-scale manufacturing, and the professional status of the performer (a pharmaceutical specialist). The Russian norm appears to have been borrowed from regulatory models of EU countries. However, in the absence of clear criteria for its application, risks of a broader interpretation arise, including the preparation of active pharmaceutical ingredients, which contradicts the objectives of patent regulation and the current structure of the pharmaceutical market.
Conclusion. Clause 5 of Article 1359 of CC RF is designed to protect pharmacy organizations and implements the principle of fair use of patented objects, provided that the medicinal products are lawfully obtained. It may serve as a negotiating tool in cases of drug shortages. To uphold constitutional obligations regarding public health protection and to eliminate legal uncertainty, the norm requires legislative clarification and refinement.
What is already known about thе subject?
► Vulvovaginal atrophy (VVA) is a common consequence of cancer treatment
► The primary focus in previous VVA research has been on estrogen deprivation and epithelial atrophy
► The role of neutrophil extracellular traps (NETs) in VVA has remained largely unexplored
What are the new findings?
► For the first time, the study identified distinct immunoinflammatory phenotypes of VVA after cancer treatment
► Chemoradiotherapy induces the strongest NETs activation (citrullinated histone H3, myeloperoxidase, cathepsin G). Antiestrogen therapy is accompanied by a higher degree of NETs activation in conditions of estrogen deprivation. “Pure” postmenopausal VVA in women without a history of cancer shows only mild NETs elevation in blood
How might it impact the clinical practice in the foreseeable future?
► NETs biomarkers may serve as tools for patient stratification and identification of therapy-resistant VVA forms. The findings support personalized treatment approaches based on immunoinflammatory phenotyping
► The data obtained provide rationale for the use of antioxidants, NETosis inhibitors, anti-protease agents, and mucosal repair strategies
► The results contribute to improved management of women after cancer treatment, where conventional therapies show the lack of efficacy in many cases
Background. Vulvovaginal atrophy (VVA) following antitumor treatment is a common and clinically significant complication. At the same time, the immunoinflammatory mechanisms determining the severity and persistence of atrophic changes are yet to be sufficiently studied. The role of extracellular neutrophil traps (NETs) in the pathogenesis of genital tract mucosal damage remains virtually unexplored.
Objective: To evaluate blood levels of NET markers – citrullinated histone H3 (CitH3), myeloperoxidase (MPO), cathepsin G (CatG) – in various VVA phenotypes after antitumor therapy and to determine their significance as potential biomarkers of atrophy severity.
Material and methods. A cross-sectional comparative study enrolled 215 postmenopausal women divided into five groups as follows: VVA after surgical treatment (n=52), chemoradiotherapy (n=27), antiestrogenic therapy (n=48), VVA without a history of cancer (n=53), and control (n=35). Clinical symptoms, vaginal pH, vaginal health index (VHI), epithelial thickness, and plasma levels of CitH3, MPO, and CatG were evaluated. Statistical analysis was performed using the Mann–Whitney test with Bonferroni correction and calculation of the r effect size.
Results. The NETs profile varied depending on the nature of the treatment received. Maximum levels of CitH3 (0,65 [0,50–0,80] ng/ml), MPO (24 [18–30] ng/ml) и CatG (14 [12–16] ng/ml) were found in women after chemoradiotherapy. Antiestrogenic therapy was accompanied by pronounced immunoinflammatory activation of NETs, while surgical menopause and VVA without a history of gynecological cancer were associated with moderate and minimal levels, respectively. Intergroup differences between the oncological groups and the control group were statistically significant (p<0.005) with a large effect size according to the Mann–Whitney criterion (r≥0.50).
Conclusion. VVA after antitumor therapy is characterized by various immunoinflammatory phenotypes, which are reflected in specific NET profiles. CitH3, MPO, and CatG can be considered pathogenetically significant markers reflecting the degree of immunoinflammatory changes in VVA, thus representing candidates for further research aimed at patient stratification and the development of personalized therapy.
REVIEW ARTICLES
What is already known about thе subject?
► Test strips are widely used to detect alcohol in saliva, ketones, glucose, cotinine, protein, bilirubin, urobilinogen, leukocytes, ascorbate and nitrites in urine, as well as substances that cause chemical dependence, a number of drugs (methamphetamine, barbiturates) and pathogens (antigens/antibodies of hepatitis viruses, influenza, COVID-19, etc.)
► Separate test systems have been developed to assess NO content in saliva, which are similar to litmus paper and allow determining pH by the color of the strip using a special color scale within 0.5–1 min
► Improvements in test strip production technology make it possible to achieve high accuracy of readings, especially when using an additional semi-automatic analyzer
What are the new findings?
► Information on using various compounds as materials for NO-sensitive and selective sensors for measuring/evaluating NO levels in various biosubstrates was systematized
► Using topological data analysis methods, an array of all currently available publications on this issue (n=1683) was studied
► The accuracy (sensitivity and selectivity) of different NO sensor systems was validated
How might it impact the clinical practice in the foreseeable future?
► The development of a reliable method for detecting NO, which would
simultaneously be characterized by high sensitivity and selectivity, biocompatibility, stability and reproducibility of results in various biosubstrates, still remains a complex scientific and technical problem
► Phthalocyanine and porphyrin-based materials can be used to develop NO sensors in aqueous solutions and biological substrates
► It is possible to use vitamin B12 derivatives as a sensitive material for NO sensors
Nitric oxide NO is a signaling molecule involved in numerous physical and pathological processes in biological systems. Highly sensitive sensor materials for measuring NO amounts in vivo in exhaled air and in body fluids (saliva, blood, urine) can be a useful tool in diagnostics and management of patients with bronchopulmonary, cardiovascular, neurological and tumor diseases. Several approaches to measuring NO in biosubstrates (including exhaled air) have been developed: fluorescence/chemiluminescence, electron spin resonance, electrochemical/amperometric (organic and inorganic) and enzymatic/protein sensors. Semiconductors, transition metal nitrides, phthalocyanine complexes, porphyrin and cobalamin derivatives with metals can serve as materials for NO sensors. Creating sensor materials based on vitamin B12 derivatives is an urgent research task in biomedicine. The article systematizes information on using various compounds as materials for NO-sensitive and selective sensors to measure/evaluate NO levels in various biosubstrates.
What is already known about thе subject?
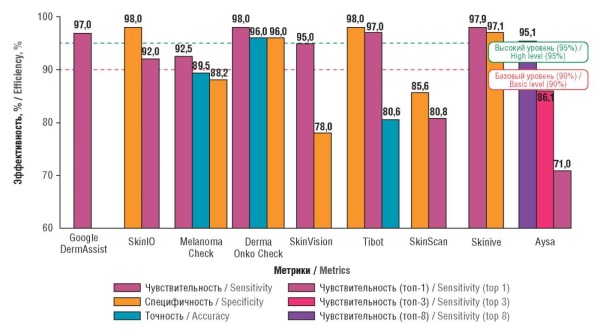
► Artificial intelligence (AI) in dermatology, especially convolutional neural networks, significantly improves the accuracy of diagnosing skin pathologies, including melanoma, with sensitivity up to 97.9% and specificity up to 98%
► Mobile apps such as Google DermAssist and Derma Onko Check demonstrate efficiency comparable to that of experienced dermatologists and can therefore be used for screening in areas with limited access to specialists
► Limitations include dependence on image quality, low accuracy on rare pathologies and dark skin tones, and the need for integration with clinical data to improve reliability
What are the new findings?
► This study presents the first original systematic comparative analysis of 9 AI-based mobile apps, conducted by independently reviewing the literature, identifying key parameters (accuracy, sensitivity, specificity) and assessing their applicability in various clinical scenarios
► Leading apps (Google DermAssist, Derma Onko Check, Skinive) with high performance and potential for use in primary health care and telemedicine were identified
► A new approach to app evaluation, systematizing differences in sensitivity (87–97.9%) and specificity (70–98%) and their impact on clinical practice was proposed
How might it impact the clinical practice in the foreseeable future?
► The analyzed AI applications could serve as second-opinion tools for general practitioners, particularly in remote areas and regions with a shortage of dermatovenereologists, thus speeding up screening and diagnosis, as well as reducing the burden on the healthcare system
► Integrating AI with telemedicine will improve personalized diagnosis and monitoring of chronic conditions such as psoriasis and eczema
► The development of algorithms for rare pathologies and dark skin tones, as well as the standardization of regulatory requirements will increase the availability and reliability of AI tools, minimizing false positives and improving clinical outcomes
Objective: To compare modern computer programs (smartphone programs – mobile applications) using artificial intelligence (AI) for diagnosing and dynamic monitoring of skin conditions.
Material and methods. A total of 1,319 publications were identified for AI-powered computer programs using targeted searches in PubMed/MEDLINE and Google Scholar databases, as well as in the eLibrary and CyberLeninka electronic libraries for the period 2016–2025. Queries focused on AI, convolutional neural networks (CNNs), computer programs (mobile apps), and dermatovenereology were used. After a multi-stage screening based on inclusion/exclusion criteria (including the availability of quantitative performance metrics), 9 key articles with specific descriptions of the computer programs (mobile apps) were selected. A search and subsequent analysis identified 9 computer programs (Google DermAssist, SkinIO, Melanoma Check, Derma Onko Check, SkinVision, Tibot, SkinScan, Aysa, and Skinive), which use AI to diagnose and monitor skin conditions.
Results. Effectiveness of the programs varies: Google DermAssist and Derma Onko Check demonstrated high accuracy (96–97%) and sensitivity (97–98%), while Skinive showed improvement in metrics over time from 2020 to 2021 (maximum sensitivity of 97.9% and specificity of 97.1%). Limitations include dependence on photo image quality, low effectiveness for rare conditions and dark skin tones, and the need for a biopsy to confirm a diagnosis. Mobile apps using CNN demonstrate high sensitivity (87–97.9%), though specificity varies significantly (70–98%), which may increase the number of additional consultations with specialist doctors when using these programs in diagnostics.
Conclusion. AI-based software (mobile apps) offers significant potential for increasing the accessibility and accuracy of skin pathology diagnostics, especially in remote areas and regions with a shortage of dermatovenereologists. Promising developments encompass the integration of computer programs with telemedicine, the refinement of algorithms for diagnosing rare pathologies, and the standardization of testing to enhance result reproducibility.
What is already known about thе subject?
► The most common clinical forms of secondary osteoarthritis (OA) are rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS)
► For the treatment of primary and secondary OA, drugs with proven symptom- and structure-modifying (disease-modifying) action should be used. These include chondroitin sulfate (CS) and glucosamine sulfate (GS)
What are the new findings?
► This systematic review presents the results of experimental and clinical studies of CS and GS in both biomodels and patients with RA, PsA, and AS, including studies of targeted biodistribution of CS and GS and molecular mechanisms of their action
How might it impact the clinical practice in the foreseeable future?
► The use of CS with a high degree of pharmaceutical standardization, proven efficacy and safety for the purpose of disease-modifying therapy of secondary OA, which is characterized by severe immunological disorders, seems relevant and promising in clinical practice
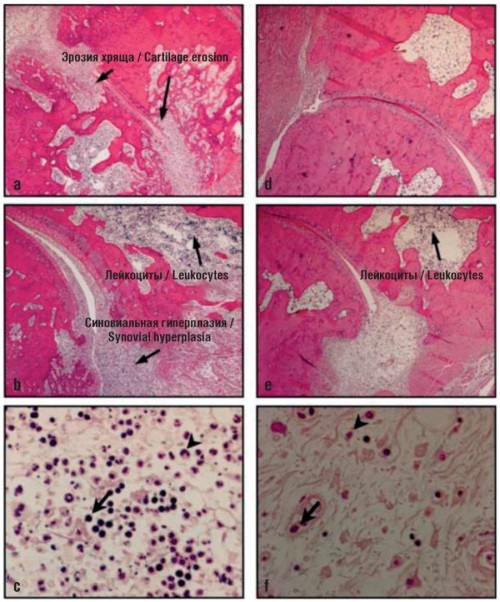
Secondary osteoarthritis (OA) may develop in the setting of various diseases and injuries of the joints, including rheumatoid arthritis (RA), psoriasis (psoriatic arthritis, PsA), and ankylosing spondylitis (AS). This article presents a systematic review of highly purified standardized drugs of chondroitin sulfate (CS) and glucosamine sulfate (GS) used in the treatment of secondary OA in various models of RA, PsA, and AS. The molecular links of targeting the pathogenesis of secondary OA are considered in specific detail. CS and GS are shown to inhibit hyperinflammation through the CD44 and TLR2/4/8 receptors, as well as the NF-κB transcription factor, thus acting as a disease-modifying therapy for RA, PsA, and AS. Experimental and clinical studies of the effects of CS and GS in RA, PsA, and AS have confirmed the prospects of their standardized forms for use in the treatment and prevention of the diseases under consideration.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
ISSN 2070-4933 (Online)