Scroll to:
Amelioration of histopathological damage in mice with induced hyperlipidemia by terpenes and phytosterols extracted from Iraqi chickpea
https://doi.org/10.17749/2070-4909/farmakoekonomika.2025.275
Abstract
Objective: To compare the amelioration effects of terpenes and phytosterols extracted from Iraqi chickpea on histopathological damage in mice fed on high-fat diet (HFD).
Material and methods. Whole chickpea plants of the Fabaceae family were collected during the flowering period in the northern area of Erbil. The collected plants were cleaned, dried in a shaded area at room temperature, pulverized with mechanical mills, and weighed. Experiments were conducted from October 2021 to April 2022. The research involved 32 healthy albino male mice 2–3 months of age, weighing about 20–30 g. The animals were provided by the Higher Institute for Diagnosis of Infertility and Assisted Reproduction Techniques. One week prior to the onset of the experiment, the animals were acclimatized to standard environmental conditions; food and water were provided ad libitum. HFD (2% cholesterol and 1% peanut butter) was added to the standard diet for 28 days to induce hyperlipidemia. In all experimental groups, the body weight was measured weekly. The terpenes and phytosterol fraction extracted from Iraqi chickpea was administered for 28 days via an intragastric tube. Mice were kept fasting for 24 hours and blood samples were extracted via heart puncture. Standard diagnostic kits and an automated analyzer were used for estimation of serum total cholesterol (TC), triglycerides (TG), low-density lipoproteins (LDL), very lowdensity lipoproteins (VLDL), high-density lipoprotein, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total serum bilirubin (TSB) levels. The animals were sacrificed, then their liver and heart organs were removed for assay.
Results. Histopathological examination of the hyperlipidemic mice group showed a marked and diffused cytoplasmic fatty infiltration of hepatic and cardiac cells. These effects were successfully ameliorated by administration of terpenes and phytosterols, although treatment with terpenes seemed to be more effective than that with phytosterols. In comparison with phytosterol treatment, terpene treatment in HFDinduced hyperlipidemic mice led to an improvement in lipid profiles, manifested in a highly significant decrease (p≤0.05) in the serum levels of TC (142.40±14.43 mg/dl), TG (98.89±8.71 mg/dl), LDL (79.43±15.14 mg/dl), and VLDL (19.78±1.74 mg/dl); with a highly significant (p≤0.05) decrease in the liver enzymatic activities of (ALT (19.19±1.36 U/l), AST (15.98±1.3 U/l), and ALP (20.99±4.43 U/l)) and TSB levels (1.60±0.12 mg/dl) (highly significantly differences with p≤0.05). Terpenes also led to a statistically significant improvement in the level of tissue malondialdehyde and glutathione in hyperlipidemic mice (p≤0.05).
Conclusion. In comparison with phytosterols, terpenes exhibit a highly significant ameliorating effect in HFD-induced hyperlipidemia, improving the state of liver and heart tissue, lipid profile, liver function enzymes, and oxidative stress parameters. Further research should assess the efficacy of terpenes and phytosterols and to elucidate the mechanism of their anti-hyperlipidemic action.
For citations:
Hwerif N.R., Abu Raghif A.R., Kadhim E.J. Amelioration of histopathological damage in mice with induced hyperlipidemia by terpenes and phytosterols extracted from Iraqi chickpea. FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology. 2025;18(1):95-103. https://doi.org/10.17749/2070-4909/farmakoekonomika.2025.275
INTRODUCTION / ВВЕДЕНИЕ
Hyperlipidemia is manifested in increased levels of cholesterol, both total cholesterol (TC) and low-density lipoproteins (LDL), oxidative stress (OS), and stimulated lipid peroxidation. Overproduction of reactive oxygen species (ROS) leads to severe cellular damage, which is primarily caused by the oxidation of cellular components including DNA and mitochondrial membrane [1]. OS has been documented to play a pivotal role in the pathophysiology and progression of diverse human diseases, including cardiovascular diseases and diabetes mellitus [2].
Hyperlipidemia can be classified as either primary or secondary, depending on the underlying reasons. Changes in lipid levels, including those found in cholesterol, triglycerides, very low-density lipoproteins (VLD), LDL, and intermediate-density lipoproteins (IDL), may lead to negative consequences in humans, such as acute pancreatitis, blood vessel blockage, and gallstone disease [3].
The selection of a suitable pharmaceutical treatment is informed by the type of lipid abnormality. Among the drugs aimed at decreasing LDL are statins, ezetimibe, resins, PCSK9 inhibitors, and niacin. Fabric acid derivatives (fibrates), niacin (nicotinic acid), and omega-3 polyunsaturated fatty acids prove effective in lowering triglyceride (TG) and VLDL concentrations and raising high-density lipoprotein (HDL) cholesterol concentrations [4]. Most of these drugs are associated with a number of side effects, including muscle-related complaints such as (myalgia, cramps, myopathy, and rhabdomyolysis), abnormal liver function, gallstone, gastric irritation. In addition, these drugs may trigger renal failure [4]. Therefore, the development of improved lipid-lowering agents alternatives to allopathic drugs represents a relevant research task [2].
Chickpea is a self-pollinating plant growing better at temperatures ranging within 15–25 °C. This plant, cultivated in more than 50 countries under varied environmental conditions [5], is one of the most consumed pulses worldwide. Chickpea is grown in the north of Iraq, as well as in Sulaimani, Kirkuk, Erbil, Duhok, and Ninawa provinces, ensuring a high quality of spring genotypes [6]. Raw or cooked chickpeas contain dietary bio-active compounds, e.g., sterols, phytic acid tannins, carotenoids, and polyphenols such as isoflavones, whose benefits may extend beyond the basic nutrition requirements of humans [7].
Terpenes are a large and diverse group of natural hydrocarbon secondary metabolites produced by a variety of plants, including tea, thyme, cannabis, conifers, and citrus fruits. Possessing five-carbon isoprene units with a chemical formula of (C5H8)n, terpenes are responsible for the fragrance, taste, and pigment of plants [8]. Certain terpenes with a set of antioxidant, anticancer, anti-inflammatory, antidiabetic, antidepressant, antiplasmodial, antimicrobial, astringent, digestive, diuretic, and other activities are widely used in natural folk medicine [8].
Phytosterols are bioactive compounds (steroids) with a chemical structure similar to that of cholesterol and are found naturally in plant species [9]. The most common dietary plant sterols are beta-sitosterol, campesterol, and stigmasterol. Phytosterols are fat-soluble compounds belonging to the triterpene family and comprising a tetracyclic ring with a side chain linked in the C17 position [10]. The health benefits of phytosterols are associated with their ability to reduce TC and LDL levels, thus mitigating the risk of many diseases [9]. Moreover, phytosterols modulate inflammation, exhibit antioxidant, antiulcer, immunomodulatory, antibacterial, and antifungal effects, and participate in wound healing and platelet aggregation inhibition [11].
Objective: To compare the effects of terpenes and phytosterols contained in Iraqi chickpea in mitigating histopathological damage in mice fed on a high-fat diet (HFD).
MATERIAL AND METHODS / МАТЕРИАЛ И МЕТОДЫ
Plant material / Растительный материал
Whole plants of chickpea of the Fabaceae family were collected during the flowering period (November 2021) in the northern area of Erbil. The collected plants were cleaned, dried in a shaded area at room temperature, pulverized with mechanical mills, and weighed.
Plant experiment work / Эксперименты c растениями
Experiments were carried out using the facilities of a phototherapy laboratory, Department of Pharmacognosy and Medicinal Plants (College of Pharmacy, University of Baghdad). The aerial parts of the plants were collected and treated as follows [12]:
– extraction and fractionation of different active constituents;
– preliminary phytochemical screening of various secondary metabolites, such as alkaloids, flavonoids, steroids, tannins, saponins, terpenoids, and cardiac glycosides in the different parts of the plant;
– isolation of sterol and terpene fractions;
– qualitative and quantitative estimation of the isolated sterols and terpenes fractions.
Animal experiments / Эксперименты с животными
The study was conducted during the period from October 2021 to April 2022 at the Department of Pharmacology (College of Medicine, Al Nahrain University). In total, 32 healthy albino male mice, 2–3 months of age and weighing about 20–30 g, were provided by the Higher Institute for Diagnosis of Infertility and Assisted Reproduction Techniques (Al Nahrain University). The animals were acclimatized to standard environmental conditions; food and water were provided ad libitum for a week prior to the commencement of the experiment.
Hyperlipidemia induction and extract administration / Индукция гиперлипидемии и введение экстракта
HFD (2% cholesterol and 1% peanut butter) was added to the standard diet (seeds, i.e., sunflower and groundnuts, cereals, fruits such as grapes and apples, vegetables, vitamins A, E, and D3) for 28 days to induce hyperlipidemia [13]. In all experimental groups, the body weight was measured weekly. Terpenes and phytosterols extracted from Iraqi chickpea were administered for 28 days via an intragastric tube [14].
Experimental design / Дизайн эксперимента
The mice were divided into four groups (8 mice in each group) [15]:
– Group 1 (normal): standard diet for 28 days;
– Group 2 (induced): HFD for 28 days;
– Group 3: HFD for 28 days followed by administration of a terpene fraction of chickpea at a dose of 250 mg/kg/day per os for further 28 days;
– Group 4: HFD for 28 days followed by administration of a phytosterol fraction of chickpea at a dose of 250 mg/kg/day per os for further 28 days.
Blood collection / Забор крови
Mice were kept fasting for 24 h, following which blood samples were extracted via heart puncture. This was followed by centrifugation at 2,500 rpm for 15 min. The obtained serum was used for the biochemical analysis of lipid profile indices and liver function enzymes [9].
Biochemical analysis / Биохимический анализ
Lipid profile indices and liver function enzymes
Standard diagnostic kits and an automated analyzer were used for estimation of serum TC, TG, LDL, VLDL, HDL, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total serum bilirubin (TSB) levels [13].
Oxidative stress parameters
At the end of the experimental period and following blood sampling, the animals were sacrificed; their liver organs were removed and divided into two parts for assay. For oxidative stress evaluation, one part of the liver tissue was homogenized and centrifuged for 15 min at 5000 rpm to assess the malondialdehyde (MDA) and glutathione (GSH) levels. Standard diagnostic kits were used to carry out an assay of MDA and GSH levels based on the competitive inhibition enzyme immunoassay technique [2].
Histopathological examination / Гистопатологическое исследование
The hepatic and heart tissue samples were first rinsed in phosphate-buffered saline, then fixed in 10% neutral buffered formalin. Standardized dehydration in ascending grades of alcohol and paraffin embedding procedures were conducted. A microtome of paraffin-embedded ultrathin sections (5 mm thickness) was carried out and slides were stained with hematoxylin and eosin. The sections were then analyzed using an Olympus light microscope equipped with a photograph machine [16].
Chickpea extraction / Процесс экстракции нута
Hexane was used for defatted oil from the plant to exclude the surplus fatty components [17] that interfere with the extraction process by adhering to the flask and biological effects inside the body1. An alcoholic solution (85% ethanol) was used. After filtration, the ethanol (crude) extract was subjected to evaporation to eliminate alcohol with the purpose of minimizing the harmful effect of ethanol on hepatic indicators. A rotary vacuum evaporator was used to avoid heat degradation of the bioactive constituents during evaporation. Methanol is a well-known solvent possessing the ability to extract semi-polar bioactive compounds [18]; hence, in the current study, it was used to extract terpenes. Petroleum ether, an organic (non-polar) solvent, was used for the extraction of phytosterols (non-polar) [19].
Statistical analysis / Статистический анализ
The Statistical Analysis System (SAS) software (SAS Institute, USA, 2018) was used to investigate the effect of different factors on the study parameters. Unpaired t-test and an analysis of variance (ANOVA) test were used to compare the parameters. The data in tables is presented as a mean value with a standard deviation (M±SD). The differences between the groups were considered statistically significant at p<0.05.
RESULTS AND DISCUSSION / РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
Serum lipid profiles / Липидные профили сыворотки крови
In the present study, the serum levels of lipid profiles of TC, TG, HDL, LDL, and VLDL were monitored, with the corresponding results being depicted in Table 1. Feeding the mice with HFD for 28 days led to a highly significant increase in serum TC, TG, LDL, and VLDL with a statistically high significant reduction in HDL in hyperlipidemic mice as compared to the group fed by a normal standard diet. The changes observed in the hyperlipidemic group may be due to a disturbance of lipid metabolism as a result of decreased β-oxidation, increased cholesterol synthesis, and oxidative stress by decreasing enzymatic-free radical scavengers [20].
Table 1. Comparison of the groups treated with terpenes (500 mg/kg/day) and phytosterols (500 mg/kg/day) with the non-treated group by ANOVA test
Taблица 1. Сравнение групп, получавших терпены (500 мг/кг/сут) и фитостерины (500 мг/кг/сут), с группой индуцированной гиперлипидемии, не получавшей лечения, по различным параметрам с помощью теста ANOVA
Parameter / Параметр | Group / Группа | |||
Normal / Контрольная (норма) | Induced (non-treated) / С индуцированной гиперлипидемией без лечения | Terpenes (500 mg/kg/day) // Лечение терпенами (500 мг/кг/сут) | Phytosterols (500 mg/kg/day) // Лечение фитостеринами (500 мг/кг/сут) | |
TC, mg/dl // Общий холестерин, мг/дл | 135.80±17.20 | 241.98±5.5 a* | 142.40±14.43 b* | 158.89±10.96 b* |
TG, mg/dl // ТГ, мг/дл | 85.92±14.04 | 183.87±13.75 a* | 98.89±8.71 b* | 107.88±15.12 b* |
VLDL, mg/dl // ЛПОНП, мг/дл | 17.18±2.81 | 36.79±2.75 a* | 19.78±1.74 b* | 21.57±3.02 b* |
LDL, mg/dl // ЛПНП, мг/дл | 67.58±19.83 | 185.03±10.03 a* | 79.43±15.14 b* | 91.85±11.75 b* |
HDL, mg/dl // ЛПВП, мг/дл | 49.37±6.10 | 16.59±5.63 a* | 43.21±3.46 b* | 45.46±7.87 b* |
ALT, U/l // АЛТ, Ед/л | 17.73±3.69 | 95.77±26.0 a* | 19.19±1.36 b* | 34.41±5.87 b* |
AST, U/l // АСТ, Ед/л | 15.33±3.83 | 196.39±53.4 a* | 15.98±1.3 b* | 20.16±2.91 b* |
ALP, U/l // ЩФ, Ед/л | 22.52±3.02 | 52.87±1.96 a* | 20.99±4.43 b* | 21.71±3.06 b* |
TSB, mg/dl // Общий билирубин, мг/дл | 1.29±0.19 | 4.87±1.10 a** | 1.60±0.12 b* | 1.74±0.31 b* |
MDA, nmol/ml // МДА, нмоль/мл | 0.53±0.23 | 4.10±1.31 a* | 0.59±0.08 b* | 0.76±0.17 b* |
GSH, U/ml // Глутатион, eд/мл | 315.77±66.97 | 57.24±19.85 a* | 298.35±38.19 b* | 265.36±10.38 b* |
Note. TC – total cholesterol; TG – triglycerides; VLDL – very low-density lipoproteins; LDL – low-density lipoproteins; HDL – high-density lipoproteins; ALT – alanine aminotransferase; AST – aspartate aminotransferase; ALP – alkaline phosphatase; TSB – total serum bilirubin; MDA – malondialdehyde; GSH – glutathione; a – comparison of the induced (non-treated) group versus the normal group; b – comparison of the terpene (500 mg/kg/day) and phytosterol (500 mg/kg/day) treated mice groups versus the induced (non-treated) group. * Statistically significant differences (p≤0.05). ** Highly statistically significant differences (p≤0.001). The differences between the phytosterol (500 mg/kg/day) treated mice group versus the terpene (500 mg/kg/day) treated mice group are not significant.
Примечание. ТГ – триглицериды; ЛПОНП – липопротеины очень низкой плотности; ЛПНП – липопротеины низкой плотности; ЛПВП – липопротеины высокой плотности; АЛТ – аланинаминотрансфераза; АСТ – аспартатаминотрансфераза; ЩФ – щелочная фосфатаза; МДА – малоновый диальдегид; а – сравнение группы мышей с индуцированной гиперлипидемией без лечения с контрольной группой; b – сравнение групп мышей, получавших терпены (500 мг/кг/сут) и фитостерины (500 мг/кг/сут) с группой с индуцированной гиперлипидемией без лечения. * Статистически значимые различия (p≤0,05). ** Высокодостоверные различия (p≤0,001). Различия между группой мышей, получавших фитостерины (500 мг/кг/сут), и группой мышей, получавших терпены (500 мг/кг/сут), не являются значимыми.
A highly significant reduction in the serum levels of lipid profile was observed among the terpene- (500 mg/kg/day) treated mice as compared to the hyperlipidemia (non-treated) mice group. These results were further supported by a significant improvement in HDL serum levels as compared to hyperlipidemia mice. According to a high-performance liquid chromatography (HPLC) analysis, the principal active constituents of terpenes are pinene, camphene, limonene, and thujene. The diversity in active constituents in the composition of terpenes impart them various pharmacological properties, such as antihyperlipidemic, anti-inflammatory, antioxidant, antidiabetes, anticancer, and antivirus [2].
A decrease in TC in the mouse group treated with of phytosterols (500 mg/kg/day) compared to the induced (non-treated) mice was noted. These results are further supported by the improved TG, HDL, LDl, and VLDL levels as compared to hyperlipidemia mice. Indeed, plant sterol lowers the absorption of cholesterol and thereby enhances the excretion of fecal steroids resulting in a reduction of the body's lipids [21]. Pancreatic lipase is considered a key enzyme responsible for TG absorption in the small intestine, and the inhibition of this enzyme by phytosterols [22] could be a key approach to controlling hyperlipidemia and obesity through suppression and delay of digestion and absorption of TG [23].
The greatest results were obtained for the terpene (500 mg/kg/day) treated mice as compared to the phytosterol (500 mg/kg/day) treated mice group in terms of serum lipid profiles.
Liver enzyme activity / Активность ферментов печени
The serum levels of ALT, AST, and ALP were significantly increased among the induced (non-treated) group, compared to the normal group. Hence, the liver function markers (ALT, AST, and ALP) as well as TSB showed a noteworthy decrease in the serum, indicating a membrane damage of hepatocytes. These consequences may be related to disturbed lipid metabolism under the action of a high fat intake, which resulted in accumulation of TG in the liver and an increase in the liver index and hepatic steatosis [24]. It is known that the liver plays a crucial role in regulating blood lipid levels through LDL clearance and HDL recruitment (see Table 1) [25].
A highly significant reduction in the serum levels of liver enzymes (ALT, AST, and ALP) followed the administration of terpenes (500 mg/kg/day), compared to the group of hyperlipidemia mice (see Table 1). Terpenes protect cells from the damage induced by oxidative stress, which is generally considered to be a cause of degenerative diseases [26]. In addition, a highly significant reduction in TSB levels indicates the liver ameliorating effects of terpenes (500 mg/kg/day).
A highly significant improvement in ALT, AST, ALP, and TSB was observed with the administration of phytosterols at a dose of 500 mg/kg/day. The underlying reason may be related to the diversity of phytochemical compounds contained in phytosterols, which possess a radical scavenging activity and hepatoprotective properties [11].
Concerning the liver enzyme activity, the terpene (500 mg/kg/day) treated mice group should more pronounced than the phytosterol (500 mg/kg/day) treated mice group.
Antioxidant activity / Антиоксидантная активность
MDA, which is a product of lipid peroxidation or oxygen reaction with unsaturated lipids, showed a highly significant increase in the group of induced (non-treated) mice [27]. The elevated levels of MDA in this group suggest an increased lipid peroxidation in fat deposits, which may have a detrimental effect on hepatic cells and other hepatocytes. In addition, the results showed a statistically highly significant reduction in tissue GSH in the induced (non-treated) group in comparison with the healthy group (see Table 1).
GSH is the most important endogenous defense mechanism against oxidative stress in the body. It plays an essential role in the maintenance of membrane protein -SH groups in the reduced form, the oxidation of which can alter cellular functions and structures [28].
A highly significant reduction of tissue MDA and an intensified defense mechanism through a highly significant increased glutathione level were noted in the group receiving terpenes (500 mg/kg) in comparison with the induced (non-treated) group (see Table 1). The antioxidant effects of terpenes are manifested through elevated catalase, superoxide dismutase, and peroxidase activities as well as through elevated levels of GSH, restoring the mitochondrial membrane [29].
A highly significant reduction of tissue MDA was also registered in the phytosterol-treated group in comparison with the induced (non-treated) group. Phytosterols act as free radical scavengers, cell membrane stabilizers, and antioxidant enzyme boosters [11]. These factors intensify the natural defense mechanism through a highly significant increase in GSH levels.
Therefore, with regard to antioxidant activity, terpene treatment (500 mg/kg/day) outperformed phytosterol treatment (500 mg/kg/day).
Histopathological examination / Гистопатологическое исследование
In the present study, the induced (non-treated) group showed a marked and diffuse cytoplasmic fatty infiltration and a granular degeneration of both hepatic (Fig. 1) and cardiac cells (Fig. 2) in comparison with the healthy (normal) mice group. This agrees well with the published data [2][30].
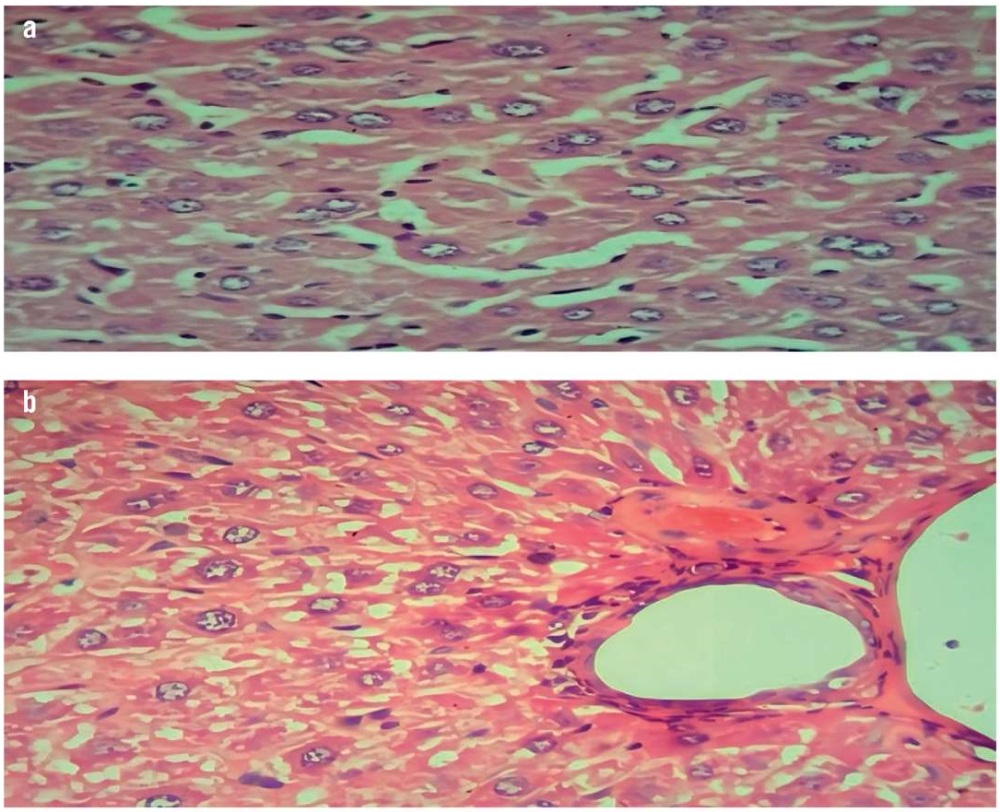
Figure 1. Histopathological examination of the mouse liver (hematoxylin and eosin staining, magnification ×40):
a – normal hepatocytes in the normal control group; b – marked and diffuse fatty steatosis with chronic inflammation of hepatocytes in the induced (non-treated) group
Рисунок 1. Патогистологическое исследование тканей печени мышей (окрашивание гематоксилином и эозином, увеличение ×40):
a – нормальные гепатоциты в контрольной группе; b – выраженный диффузный жировой стеатоз с хроническим воспалением гепатоцитов в группе индуцированной гиперлипидемии без лечения
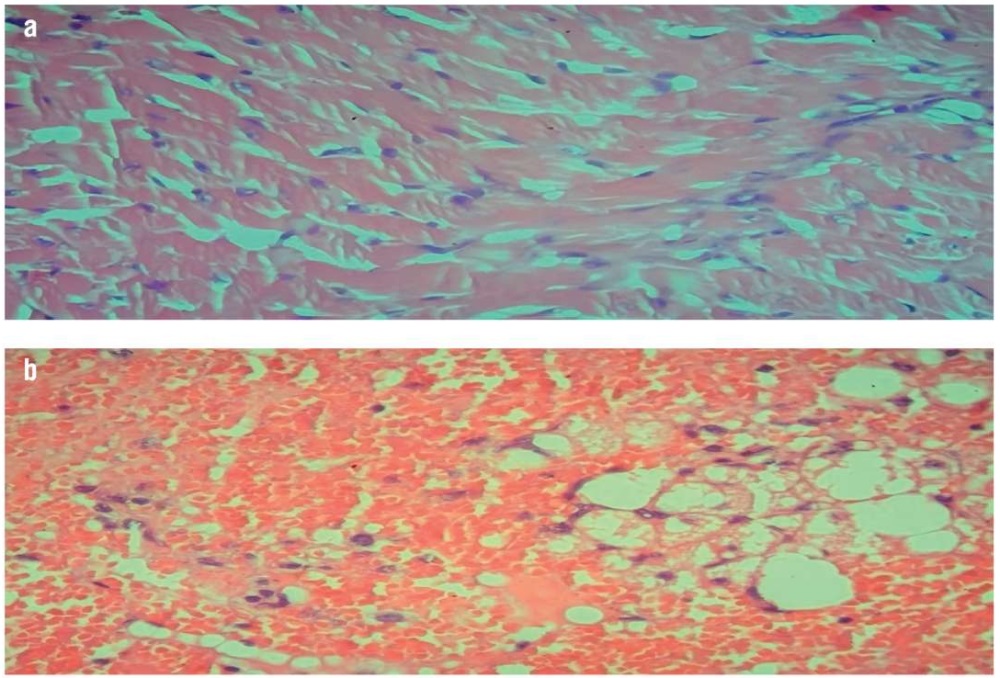
Figure 2. Histopathological examination of the mouse heart (hematoxylin and eosin staining, magnification ×40):
a – normal cardiac muscle with centrally arranged nuclei in the normal control group; b – marked fat deposition, neutrophilic cell infiltration, and vascular congestion in the induced (non-treated) group
Рисунок 2. Патогистологическое исследование тканей сердца мышей (окрашивание гематоксилином и эозином, увеличение ×40):
a – нормальная сердечная мышца с центрально расположенными ядрами в контрольной группе; b – выраженное жироотложение, инфильтрация нейтрофильными клетками и закупорка сосудов в группе индуцированной гиперлипидемии без лечения
The results obtained showed a significant improvement in the state of hepatocytes and cardiomyocytes among the terpene (500 mg/kg/day)-treated mice group in comparison with the induced (non-treated) mice group (Fig. 3). These findings indicate the antihyperlipidemic and anti-inflammatory effects of terpenes, which may be exerted through inhibited pro-inflammatory cytokines similar to those occurring with limonene pre-treatment [31]. In addition, the effect of lipid metabolism may be due to the presence of camphene, which was previously reported to prevent hepatic steatosis and to exhibit a hypolipidemic effect in high-fat diet mice [32].
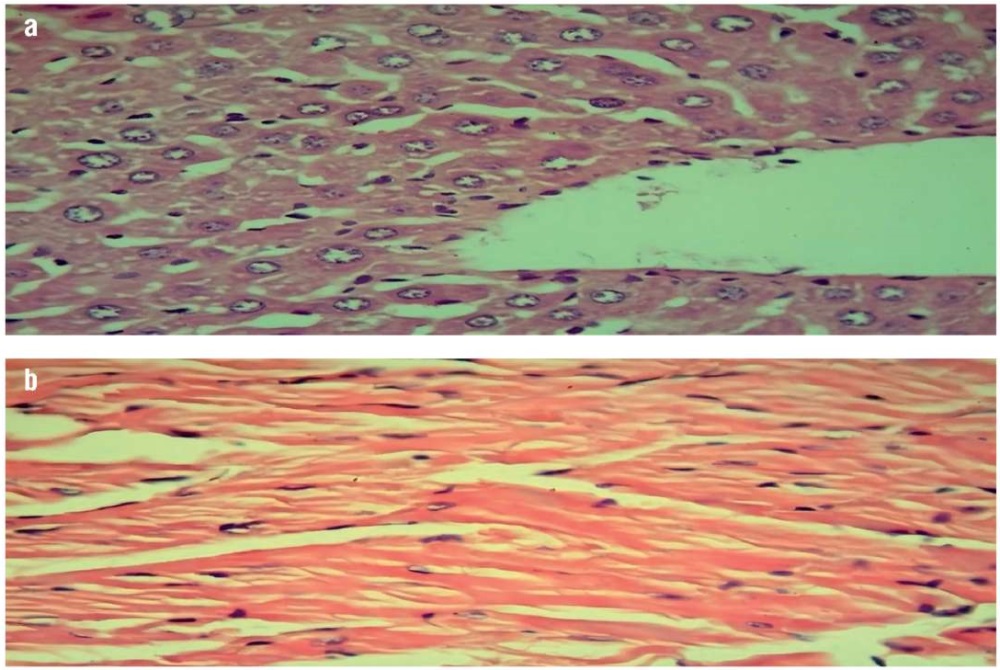
Figure 3. Histopathological examinations of mice treated with terpenes (500 mg/kg/day) (hematoxylin and eosin staining, magnification ×40):
a – liver examination showing normal hepatocytes with scattered fatty steatosis; b – heart examination showing normal cardiac muscle
Рисунок 3. Патогистологическое исследование тканей мышей, получавших терпены (500 мг/кг/сут) (окрашивание гематоксилином и эозином, увеличение ×40):
a – исследование печени показало нормальные гепатоциты с рассеянным жировым стеатозом; b – исследование сердца выявило нормальную сердечную мышцу
In addition, our study revealed an improvement in the histopathological pictures of the liver and heart tissue in the phytosterol (500 mg/kg/day) treated mice group (Fig. 4). This indicates the antihyperlipidemic and anti-inflammatory effects of phytosterols, which may be due to reduced macrophage and neutrophil-mediated inflammatory processes and pro-inflammatory cytokine concentrations [33].
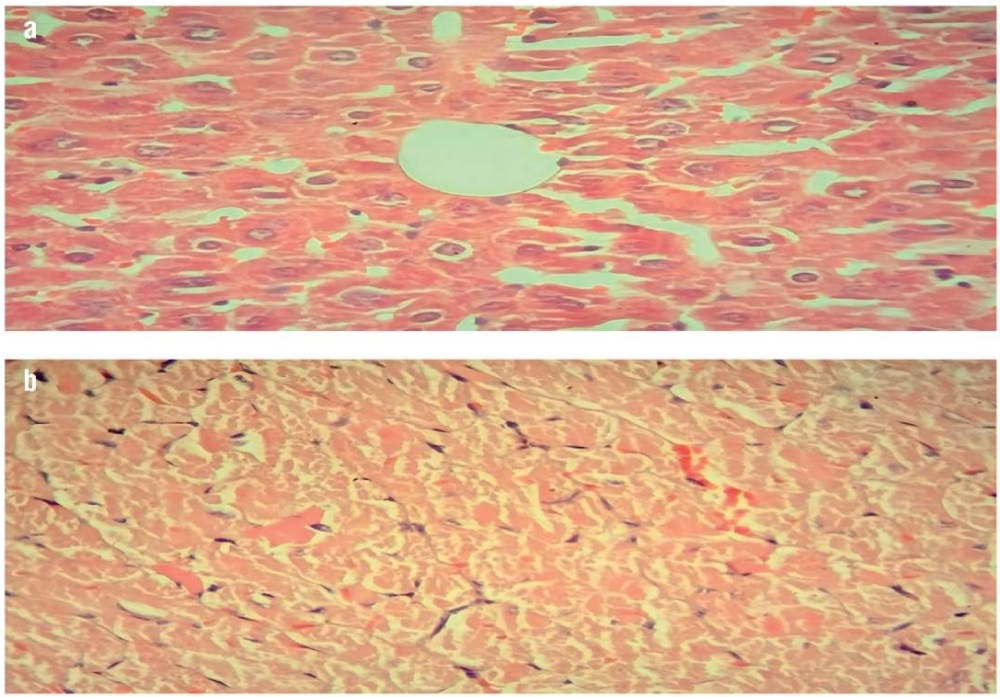
Figure 4. Histopathological examination of mice treated with phytosterols (500 mg/kg/day) (hematoxylin and eosin staining, magnification ×40):
a – liver examination showing mild fatty steatosis and scattered mild degenerative changes; b – heart examination showing normal cardiac muscle and mild fat deposition with vascular congestion
Рисунок 4. Патогистологическое исследование тканей мышей, получавших фитостерины (500 мг/кг/сут) (окрашивание гематоксилином и эозином, увеличение ×40):
a – исследование печени выявило умеренный жировой стеатоз и рассеянные незначительные дегенеративные изменения; b – исследование сердца показало нормальную сердечную мышцу и небольшое отложение жира с закупоркой сосудов
The mice treated with phytosterols (500 mg/kg/day) showed an improvement in the cellular histoarchitectural morphology, while the terpene (500 mg/kg/day) treated mice showed a distinct quasi-normal histopathological profile.
Research prospects / Перспективы исследований
The potential challenges in translating the results obtained in mouse models to human studies include the bioavailability, pharmacokinetics, and side effects of the studied substances. Their further research in terms of efficacy and safety is required.
The investigated absorption-related fractions alter stability and solubility; as a result, such variables as enzymes, transporters, and natural flora influence absorption and metabolism. Therefore, absorption is a critical aspect in determining the efficacy of the studied substances in people. Their solubility should be improved using modern approaches. The metabolism of phytochemicals can differ dramatically between species, resulting in distinct bioactive metabolite profiles. As a result, these substances have an effect on metabolism, which is important for determining the rate of terpene and phytosterol metabolizing enzymes. Phytosterols and terpenes may impact the first-pass hepatic metabolism (bioavailability).
The available information shows that phytosterol supplements are reasonably safe and well-tolerated. The reported side effects are, as a rule, modest and include constipation, nausea, upset stomach, heartburn, gas, and stool discoloration. Chickpea-derived compounds may cause an allergic response. Hormonal alterations should be observed due to the similarity of phytosterol molecules.
CONCLUSION / ЗАКЛЮЧЕНИЕ
The terpene fraction (500 mg/kg/day per os) of Iraqi chickpea demonstrated highly significant antihyperlipidemic, anti-inflammatory, and antioxidant effects. The diversity in the presence of phytochemical compounds, such as pinene, camphene, limonene, and thujene, may be responsible for the inhibition of lipid peroxidation.
The phytosterol fraction (500 mg/kg/day per os) of Iraqi chickpea demonstrated highly significant antioxidant, antihyperlipidemic, and anti-inflammatory effects. The diversity in the presence of phytochemical compounds, such as beta-sitosterol, campesterol, and stigmasterol may be responsible for the inhibition of lipid peroxidation.
The effects of terpenes (500 mg/kg/day) and phytosterols (500 mg/kg/day) extracted from Iraqi chickpea were comparable in different parameters. Terpenes (500 mg/kg/day) showed the highest results concerning histopathology outcomes and led to a significant improvement of serum lipid profiles, liver enzyme activity, and antioxidant activities. Although no significant differences were observed between terpenes (500 mg/kg/day) and phytosterols (500 mg/kg/day) in increasing the levels of HDL, phytosterols demonstrated better results.
1. Al-Hakeemi A. Isolation of Trigonelline from Iraqi Fenugreek seed and studying its effect on blood glucose level and lipid profile in normal and alloxan-diabetic rabbits. M. Sc. Thesis; 2002.
References
1. Abu-Raghif A.R., Sahib H.B., Abbas S.N. Anti-hyperlipidemic effect of Vitex agnus castus extracts in mice. Int J Pharm Sci Rev Res. 2015; 35 (2): 120–5.
2. Hwerif N., Raghif A., Kadhim E. Effect of terpenes fraction of Iraqi cicer arietinum in experimentally induced hyperlipidemic mice. Int J Health Sci. 2022; 6 (S5): 10514–30. https://doi.org/10.53730/ijhs.v6nS5.11275.
3. Abbas S.N., Shihab E.M., Shareef S.M., et al. Effect of bosentan in experimentally induced hyperlipidemic mice. J Population Ther Clin Pharmacol. 2023; 30 (9): e231–8.
4. Ward N.C., Pang J., Ryan J.D., Watts G.F. Nutraceuticals in the management of patients with statin-associated muscle symptoms, with a note on real-world experience. Clin Cardiol. 2018; 41 (1): 159–65. https://doi.org/10.1002/clc.22862.
5. Acevedo Martinez K.A., Yang M.M., Gonzalez de Mejia E. Technological properties of chickpea (Cicer arietinum): production of snacks and health benefits related to type-2 diabetes. Compr Rev Food Sci Food Saf. 2021; 20 (4): 3762–87. https://doi.org/10.1111/1541-4337.12762.
6. Hamarash A.M. Study the phnotypic and productive characters of seven selected genotypes of chickpea (Cicer arietinum L.) cv. (Kabuli and Desi) under rainfall conditions in Sulaimani Province, Iraq. Kufa Journal for Agricultural Sciences. 2020; 12 (2): 43–53.
7. Wallace T.C., Murray R., Zelman K.M. The nutritional value and health benefits of chickpeas and hummus. Nutrients. 2016; 8 (12): 766. https://doi.org/10.3390/nu8120766.
8. Cox-Georgian D., Ramadoss N., Dona C., Basu C. Therapeutic and medicinal uses of terpenes. Medicinal Plants. 2019 Nov 12: 333–59. https://doi.org/10.1007/978-3-030-31269-5_15.
9. Hwerif N.R., Raghif A.R.A., Kadhim E.J. Effect of phytosterols fraction of Iraqi cicer arietinum in experimentally induced hyperlipidemic mice. AMJ. 2022; 62 (06): 2677–92.
10. Moreau R.A., Nyström L., Whitaker B.D., et al. Phytosterols and their derivatives: structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog Lipid Res. 2018; 70: 35–61. https://doi.org/10.1016/j.plipres.2018.04.001.
11. Salehi B., Quispe C., Sharifi-Rad J., et al. Phytosterols: From preclinical evidence to potential clinical applications. Front Pharmacol. 2021; 11: 599959. https://doi.org/10.3389/fphar.2020.599959.
12. Khadim E.J., Abdulrasool A.A., Awad Z.J. Phytochemical investigation of alkaloids in the Iraqi Echinops heterophyllus (Compositae). Iraqi J Pharm Sci. 2014; 23 (1): 26–34.
13. Coakly W.A. Handbook of automated analysis: continuous flow technique. Mercel Dekker; 1981: 61 pp.
14. Wang L.X., Liu K., Gao D.W., Hao J.K. Protective effects of two Lactobacillus plantarum strains in hyperlipidemic mice. World J Gastroenterol. 2013; 19 (20): 3150–6. https://doi.org/10.3748/wjg.v19.i20.3150.
15. Pandit K., Karmarkar S., Bhagwat A. Evaluation of antihyperlipidemic activity of Ficus hispida linn leaves in Triton WR-1339 (Tyloxapol) induced hyperlipidemia in mice. Int J Pharm Pharm Sci. 2011; 3 (Suppl. 5): 188–91.
16. Bancroft J.D., Gamble M. Theory and practice of histological techniques. 6th ed. Churchill Livingstone; 2007: 744 pp.
17. Prasanna M. Hypolipidemic effect of fenugreek: a clinical study. Indian J Pharmacol. 2000; 32 (1): 34–6.
18. Meier B. From medicinal plant to phytotherapeutic drug. Ther Umsch. 2002; 59 (6): 275–82 (in German). https://doi.org/10.1024/0040-5930.59.6.275.
19. Garcia-Llatas G., Alegria A., Barberá R., Cilla A. Current methodologies for phytosterol analysis in foods. Microchem J. 2021; 168: 106377. https://doi.org/10.1016/j.microc.2021.106377.
20. Yang R.L., Li W., Shi Y.H., Le G.W. Lipoic acid prevents high-fat dietinduced dyslipidemia and oxidative stress: a microarray analysis. Nutrition. 2008; 24 (6): 582–8. https://doi.org/10.1016/j.nut.2008.02.002.
21. Ghule B., Ghante M., Saoji A., Yeole P. Hypolipidemic and antihyperlipidemic effects of Lagenaria siceraria (Mol.) fruit extracts. Indian J Exp Biol. 2006; 44 (11): 905–9.
22. Lim S.M., Goh Y.M., Kuan W.B., Loh S.P. Effect of germinated brown rice extracts on pancreatic lipase, adipogenesis and lipolysis in 3T3-L1 adipocytes. Lipids Health Dis. 2014; 13: 169. https://doi.org/10.1186/1476-511X-13-169.
23. Zhang B., Deng Z., Ramdath D.D., et al. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015; 172: 862–72. https://doi.org/10.1016/j.foodchem.2014.09.144.
24. Yin Y., Yu Z., Xia M., et al. Vitamin D attenuates high fat dietinduced hepatic steatosis in rats by modulating lipid metabolism. Eur Clin Invest. 2012; 42 (11): 1189–96. https://doi.org/10.1111/j.1365-2362.2012.02706.x.
25. Preiss D., Sattar N. Non-alcoholic fatty liver disease: an overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin Sci. 2008; 115 (5): 141–50. https://doi.org/10.1042/CS20070402.
26. Kim I.S., Yang M.R., Lee O.H., Kang S.N. Antioxidant activities of hot water extracts from various spices. Int J Mol Sci. 2011; 12 (6): 4120–31. https://doi.org/10.3390/ijms12064120.
27. Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014; 2014: 360438. https://doi.org/10.1155/2014/360438.
28. Pandey K.B., Rizvi S.I. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010; 3 (1): 2–12. https://doi.org/10.4161/oxim.3.1.10476.
29. Kim T., Song B., Cho K.S., Lee I.S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int J Mol Sci. 2020; 21 (6): 2187. https://doi.org/10.3390/ijms21062187.
30. Chaiwong S., Chatturong U., Chanasong R., et al. Dried mulberry fruit ameliorates cardiovascular and liver histopathological changes in high-fat diet-induced hyperlipidemic mice. J Tradit Complement Med. 2021; 11 (4): 356–68. https://doi.org/10.1016/j.jtcme.2021.02.006.
31. Chi G., Wei M., Xie X., et al. Suppression of MAPK and NF-κB pathways by limonene contributes to attenuation of lipopolysaccharideinduced inflammatory responses in acute lung injury. Inflammation. 2013; 36 (2): 501–11. https://doi.org/10.1007/s10753-012-9571-1.
32. Vallianou I., Hadzopoulou-Cladaras M. Camphene, a plant derived monoterpene, exerts its hypolipidemic action by affecting SREBP-1 and MTP expression. PLoS One. 2016; 11 (1): e0147117. https://doi.org/10.1371/journal.pone.0147117.
33. Brüll F., Mensink R. Plant sterols: functional lipids in immune function and inflammation? Clin Lipidol. 2009; 4 (3): 355. https://doi.org/10.2217/clp.09.26.
About the Authors
N. R. HwerifIraq
Nihal Ramadhan Hwerif
Kadhimiya-street 60 Imam Khadhimin Medical City, Baghdad
A. R. Abu Raghif
Iraq
Ahmed Rahmah Abu Raghif
Scopus Author ID: 8950029500
Kadhimiya-street 60 Imam Khadhimin Medical City, Baghdad
E. J. Kadhim
Russian Federation
Enas Jawad Kadhim
Scopus Author ID: 56149522600
Bab Al Mu'adham, Baghdad
What is already known about thе subject?
► Terpenes and phytosterols exhibit anti-hyperlipidemic effects by reducing cholesterol absorption, improving lipid metabolism, and adjusting lipoprotein levels
► Clinical studies showed that phytosterols are capable of reducing low-density lipoprotein levels by 5–15%, with a higher intake resulting in a larger decrease. They may lead to a slightly decrease in triglyceride levels, particularly in hypertriglyceridemia
What are the new findings?
► The anti-inflammatory, antioxidant, and anti-hyperlipidemic properties of terpenes and phytosterols extracted from Iraqi chickpeas were evaluated in animal experiments, revealing their potential as bioactive substances
► Histopathological examination showed significant improvement in the state of hepatocytes and cardio-myocytes in the mice groups receiving terpenes and phytosterols in comparison with the non-treated mice group
How might it impact the clinical practice in the foreseeable future?
► The use of terpenes and phytosterols from Iraqi chickpeas could significantly impact therapeutic practice, potentially leading to new treatment approaches for hyperlipidemia, cardiovascular conditions, and metabolic illnesses
► Terpenes and phytosterols contained in Iraqi chickpea could be introduced in functional meals or supplements to treat hyperlipidemia and associated disorders; to that end, further clinical trials involving humans are required
Review
For citations:
Hwerif N.R., Abu Raghif A.R., Kadhim E.J. Amelioration of histopathological damage in mice with induced hyperlipidemia by terpenes and phytosterols extracted from Iraqi chickpea. FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology. 2025;18(1):95-103. https://doi.org/10.17749/2070-4909/farmakoekonomika.2025.275

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






































